Finding Mass of Atoms (Experimentally)
We are given the molecular masses of 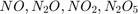 , located in exp_values.
, located in exp_values.
% In matrix form A = [1 1 2 1 1 2 2 5]; % Experimental exp_values = [30.006; 44.013; 46.006; 108.010]; % The answers x = A\exp_values
x = 14.0069 15.9993
Thus, the atomic mass of N is 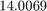 and O is
and O is 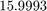 .
.